In the intricate world of electronics, printed circuit boards (PCBs) serve as the backbone of countless devices we rely on daily. These complex networks of conductive pathways and components are marvels of engineering, but they face a persistent enemy: corrosion. The gradual degradation of metal components on PCBs can lead to device malfunction, reduced performance, and ultimately, failure. As our reliance on electronic devices continues to grow, understanding how to effectively clean and prevent corrosion on circuit boards has become increasingly crucial.
Corrosion on PCBs is not merely a cosmetic issue; it’s a serious threat to the functionality and longevity of electronic devices. From smartphones to industrial control systems, the impact of corrosion can be far-reaching and costly. This article delves into the intricacies of PCB corrosion, exploring its causes, types, and most importantly, how to combat it effectively. We’ll examine the science behind corrosion, the tools and techniques for cleaning affected boards, and strategies for preventing future damage.
What is PCB Corrosion
PCB corrosion is a complex electrochemical process that occurs when the metal components of a printed circuit board react with their environment, leading to degradation of the conductive pathways and potential failure of the electronic device. At its core, corrosion is the result of oxidation, a chemical reaction where metal atoms lose electrons to oxygen molecules in the presence of an electrolyte, typically water or moisture in the air.
The process of corrosion on PCBs is particularly insidious because it can begin almost imperceptibly and progress rapidly under certain conditions. When oxygen bonds with metal surfaces on a circuit board, it creates metal oxides, commonly known as rust. This oxidation process causes the metal to flake off, compromising the integrity of the conductive traces and solder joints that are crucial for the board’s functionality.
One of the most significant impacts of PCB corrosion is the loss of electrical conductivity. As corrosion progresses, the resistance of affected traces increases, leading to slower signal propagation and reduced operating speeds. In severe cases, corrosion can cause complete breaks in the conductive pathways, resulting in open circuits and device failure. Moreover, the accumulation of corrosion products can bridge adjacent traces, potentially causing short circuits and further damage to the board or connected components.
It’s important to note that not all metals used in PCB manufacturing are equally susceptible to corrosion. Noble metals like gold and silver exhibit high resistance to corrosion, which is why they are often used for critical connections or as protective plating. Copper, the most common material for PCB traces, is relatively resistant to corrosion under normal conditions but can degrade rapidly in harsh environments or when exposed to certain contaminants. Other metals commonly found on PCBs, such as lead in solder joints or nickel in plating, have varying degrees of corrosion resistance.
The impact of corrosion on electronic devices can be profound. As corrosion progresses, devices may experience intermittent failures, reduced performance, or complete malfunction. In sensitive applications, such as medical devices or aerospace electronics, even minor corrosion can have serious consequences. The financial implications of PCB corrosion are also significant, with industries spending billions annually on corrosion prevention and remediation.
Understanding PCB corrosion requires consideration of various factors, including the materials used in board construction, environmental conditions, and the presence of contaminants. Humidity, temperature fluctuations, and exposure to corrosive substances can all accelerate the corrosion process. Additionally, manufacturing defects, such as incomplete solder mask coverage or residual flux, can create vulnerabilities that make PCBs more susceptible to corrosion.
Types of PCB Corrosion
PCB corrosion manifests in various forms, each with its unique characteristics and challenges:
Atmospheric Corrosion
Atmospheric corrosion is the most prevalent form of PCB degradation, occurring when metal components are exposed to moisture and oxygen in the air. This type of corrosion is particularly problematic for copper traces, which are ubiquitous in PCB design. The process begins with the formation of a thin film of moisture on the metal surface, which acts as an electrolyte. Oxygen then diffuses through this film, reacting with the metal to form oxides.
In the case of copper, the initial corrosion product is often copper(I) oxide (Cu2O), which appears as a reddish-brown layer. Over time, this can further oxidize to copper(II) oxide (CuO), which is black. While these oxide layers can provide some protection against further corrosion, they also increase electrical resistance and can lead to connectivity issues.
Atmospheric corrosion is exacerbated by environmental factors such as high humidity, temperature fluctuations, and the presence of airborne pollutants. In coastal areas, for example, the salt content in the air can significantly accelerate corrosion rates. Industrial environments with high levels of sulfur dioxide or other corrosive gases also pose a significant risk to PCBs.
Galvanic Corrosion
Galvanic corrosion occurs when two dissimilar metals are in electrical contact in the presence of an electrolyte. This type of corrosion is particularly insidious in PCB design because it can occur even when the board is not powered. The difference in electrochemical potential between the metals creates a galvanic cell, where the more active metal (anode) corrodes preferentially to protect the more noble metal (cathode).
A common example of galvanic corrosion in PCBs is the interaction between gold-plated connectors and the underlying copper or nickel substrate. If the gold plating is thin or damaged, exposing the base metal, corrosion can occur rapidly in the presence of moisture. This not only compromises the integrity of the connection but can also lead to the formation of non-conductive corrosion products that interfere with electrical contact.
Electrolytic Corrosion
Electrolytic corrosion, also known as electrochemical migration, is a form of corrosion that occurs when an electric field is present between adjacent conductors in the presence of an electrolyte. This type of corrosion is particularly problematic in high-density PCB designs where traces are closely spaced.
The process begins with the dissolution of metal ions from the anode (positively charged conductor). These ions then migrate through the electrolyte towards the cathode (negatively charged conductor). As they travel, they can form conductive dendrites – tree-like structures that grow from the cathode towards the anode. If these dendrites bridge the gap between conductors, they can cause short circuits and device failure.
Electrolytic corrosion is often exacerbated by contamination on the PCB surface, such as flux residues or other ionic species that increase the conductivity of any moisture present. It can occur rapidly, sometimes within hours of exposure to moisture, making it a significant concern in high-reliability applications.
Fretting Corrosion
Fretting corrosion is a unique form of degradation that occurs at the interface of two contacting surfaces subjected to slight relative motion. In PCBs, this type of corrosion is commonly seen in connectors, particularly those that experience vibration or thermal cycling.
The process begins with mechanical wear of the protective oxide layer on the metal surface. This exposes fresh metal, which quickly oxidizes. The resulting oxide particles are abrasive, leading to further wear and corrosion. Over time, this can result in increased contact resistance, intermittent connections, or complete failure of the electrical contact.
Fretting corrosion is particularly problematic in applications where PCBs are subjected to vibration, such as automotive or aerospace electronics. It can also occur in devices that undergo frequent thermal cycling, as the expansion and contraction of materials can cause slight movements at contact points.
Pitting Corrosion
Pitting corrosion is a localized form of corrosion that results in the formation of small holes or pits in the metal surface. This type of corrosion is particularly dangerous because it can penetrate deep into the metal while leaving the surrounding area relatively unaffected, making it difficult to detect visually.
In PCBs, pitting corrosion often occurs in areas where the protective coating (such as solder mask) has been damaged or where contaminants have accumulated. It can be initiated by the presence of chloride ions, which are common in many environments. Once a pit starts to form, it can create a self-sustaining corrosion cell, with the pit acting as the anode and the surrounding metal surface as the cathode.
Pitting corrosion can lead to rapid failure of PCB traces or pads, as the cross-sectional area of the conductor is reduced. In severe cases, it can completely penetrate through the copper layer, creating open circuits.
Causes of PCB Corrosion
The corrosion of printed circuit boards is a complex phenomenon influenced by a multitude of factors:
Environmental Factors
Humidity is perhaps the most significant environmental factor, as it provides the moisture necessary for many corrosion reactions to occur. When relative humidity exceeds 60%, a thin film of water can form on metal surfaces, creating an electrolyte that facilitates corrosion processes.
Temperature also plays a crucial role, with higher temperatures generally accelerating corrosion rates. However, it’s not just high temperatures that pose a risk; temperature fluctuations can be equally problematic. Thermal cycling can lead to condensation when warm, humid air comes into contact with cooler surfaces, creating ideal conditions for corrosion. Additionally, temperature changes can cause mechanical stress due to the different thermal expansion rates of materials used in PCB construction, potentially exposing vulnerable areas to corrosive elements.
Airborne pollutants and chemicals in the environment can significantly exacerbate corrosion. Industrial environments, for instance, may contain sulfur dioxide, nitrogen oxides, or chlorine compounds that can react with moisture to form highly corrosive acids. Coastal areas present a unique challenge due to the presence of salt in the air, which can accelerate corrosion rates dramatically. Even in seemingly benign office environments, ozone from electronic equipment and volatile organic compounds from cleaning products can contribute to corrosion over time.
Manufacturing and Design Issues
Poor quality control during production can lead to a variety of issues that make boards more susceptible to corrosion. For example, inadequate cleaning after soldering can leave flux residues on the board, which can attract moisture and become corrosive over time.
The choice of materials used in PCB construction is critical. While copper is the most common material for traces due to its excellent conductivity and relatively good corrosion resistance, it can still be vulnerable under certain conditions. The quality of the copper used, including its purity and grain structure, can affect its corrosion resistance. Similarly, the choice of solder mask and other protective coatings can significantly impact a board’s ability to withstand corrosive environments.
Design flaws can also contribute to corrosion issues. Inadequate spacing between traces can increase the risk of electrolytic corrosion, while sharp corners in trace design can create stress points that are more susceptible to corrosion. Furthermore, the layout of components on the board can create areas where moisture or contaminants can accumulate, increasing the risk of localized corrosion.
Device Usage and Maintenance
The way electronic devices are used and maintained can significantly impact their susceptibility to corrosion. Exposure to liquids is one of the most common causes of PCB corrosion in consumer electronics. Spills, high humidity environments, or even condensation from rapid temperature changes can introduce moisture to the board, initiating corrosion processes.
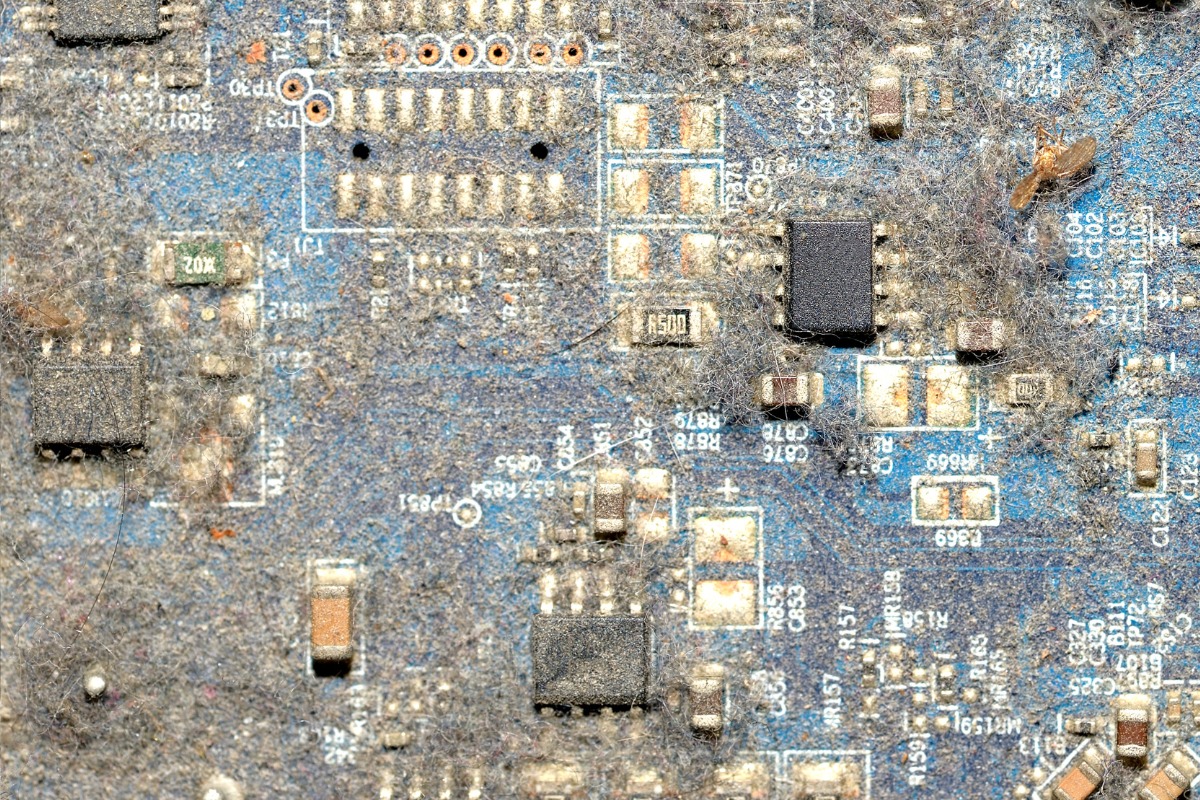
Accumulation of dust and debris on PCBs can exacerbate corrosion in several ways. Dust can be hygroscopic, meaning it absorbs moisture from the air, creating a localized high-humidity environment on the board surface. Additionally, some types of dust can be conductive or contain corrosive elements, further compromising the board’s integrity.
Lack of regular cleaning and maintenance can allow corrosion to progress unchecked. In industrial or harsh environments, regular inspection and cleaning of PCBs can be crucial for early detection and prevention of corrosion issues. However, it’s important to note that improper cleaning techniques or the use of inappropriate cleaning agents can sometimes do more harm than good, potentially introducing contaminants or damaging protective coatings.
Component Failures
The failure of individual components on a PCB can lead to corrosion issues that affect the entire board. Leaking batteries are a prime example of this. When a battery leaks, it can release corrosive electrolytes onto the PCB, causing rapid and severe corrosion. This is particularly problematic in devices with built-in batteries or those left unused for extended periods.
Faulty capacitors can also contribute to corrosion problems. Electrolytic capacitors, in particular, contain a liquid electrolyte that can leak if the capacitor is damaged or reaches the end of its lifespan. This electrolyte is often corrosive and can damage nearby components and traces.
Electrochemical Factors
The fundamental nature of PCBs, with their network of conductive traces carrying various voltages, creates an environment ripe for electrochemical corrosion. When moisture or other electrolytes are present on the board surface, voltage differences between adjacent traces can drive corrosion reactions. This is particularly problematic in high-density designs where traces are closely spaced.
The presence of contaminants can exacerbate these electrochemical processes. Ionic contaminants, which can come from flux residues, fingerprints, or environmental pollutants, increase the conductivity of any moisture present on the board. This enhanced conductivity accelerates corrosion reactions and can lead to the formation of conductive dendrites between traces.
Time and Cumulative Effects
It’s important to recognize that corrosion is often a gradual process, with damage accumulating over time. While some forms of corrosion can occur rapidly under extreme conditions, in many cases, the effects of corrosion may not become apparent until months or years after the initial exposure to corrosive conditions.
This cumulative nature of corrosion damage underscores the importance of proactive prevention measures. By the time visible signs of corrosion appear, significant damage may have already occurred at a microscopic level. Understanding this time-dependent aspect of corrosion is crucial for developing effective long-term protection strategies for PCBs.
Materials and Tools for Cleaning Corrosion
Effectively cleaning corrosion from printed circuit boards requires a careful selection of materials and tools. The choice of cleaning agents and implements can significantly impact the success of the cleaning process and the long-term health of the PCB.
Cleaning Solutions
The cornerstone of any PCB cleaning process is the choice of cleaning solution. Different types of corrosion and contaminants may require specific cleaning agents. Here are some of the most commonly used and effective cleaning solutions:
Isopropyl Alcohol (IPA)
Isopropyl alcohol, particularly in concentrations of 90% or higher, is a versatile and widely used cleaning agent for PCBs. Its rapid evaporation and ability to leave no residue make it ideal for removing light corrosion, flux residues, and general contaminants. IPA is effective at dissolving many organic compounds and can help in removing oils and greases that may have accumulated on the board surface. When using IPA, it’s important to note that while it’s relatively safe, prolonged exposure can cause drying and irritation of the skin. Always use IPA in a well-ventilated area and wear appropriate personal protective equipment, including gloves and eye protection.
Distilled or Deionized Water
Pure water, free from ions and minerals, is an essential component of many cleaning processes. Unlike tap water, which contains dissolved minerals that can leave conductive residues on the board, distilled or deionized water evaporates cleanly. It’s particularly useful for rinsing boards after using other cleaning agents and for diluting concentrated cleaning solutions. The use of pure water is crucial because any residual ions left on the board surface can contribute to future corrosion or affect the board’s electrical properties. When using water in PCB cleaning, always ensure it’s of high purity to avoid introducing new contaminants.
Baking Soda
Sodium bicarbonate, commonly known as baking soda, is an excellent mild abrasive and alkaline substance that can be effective in neutralizing acidic corrosion products. Its gentle abrasive properties make it useful for removing stubborn corrosion without damaging the underlying metal or board substrate. To use baking soda for cleaning, it’s typically mixed with a small amount of water to form a paste. This paste can be applied to corroded areas and gently worked with a soft brush. The alkaline nature of baking soda helps to neutralize acidic corrosion products, while its mild abrasiveness aids in mechanical removal of corrosion.
White Vinegar
While it should be used with caution, white vinegar (dilute acetic acid) can be effective for dissolving certain types of corrosion, particularly those involving alkaline compounds. Its acidic nature can help break down corrosion products that are resistant to other cleaning methods. However, it’s crucial to use vinegar judiciously and with expert guidance. The acidity of vinegar can potentially cause further corrosion if not used correctly or if not thoroughly rinsed and neutralized after use. Always dilute vinegar with distilled water and limit exposure time to minimize risks.
Cleaning Tools
The right tools are essential for applying cleaning solutions effectively and safely. Here are some key implements used in PCB corrosion cleaning:
Soft-bristled Brushes
Gentle brushing is often necessary to dislodge corrosion products and work cleaning solutions into affected areas. Soft-bristled brushes, such as old toothbrushes or specialized ESD-safe PCB cleaning brushes, are ideal for this purpose. The soft bristles help prevent scratching of the board surface or damage to delicate components. When selecting a brush, consider the density and arrangement of components on the board. Smaller, more precise brushes may be necessary for working around densely packed surface-mount components.
Cotton Swabs
For precise application of cleaning solutions and for cleaning in tight spaces between components, cotton swabs are invaluable. They allow for targeted cleaning of small areas and can be easily disposed of after use, preventing cross-contamination. When using cotton swabs, be mindful of loose fibers that may detach and remain on the board. Always inspect the cleaned area carefully and use compressed air to remove any residual fibers.
Lint-free Cloths or Microfiber Towels
For final cleaning and drying of PCBs, lint-free cloths or microfiber towels are essential. These materials are designed to clean effectively without leaving behind fibers or particles that could interfere with board function. Microfiber towels are particularly effective due to their ability to trap small particles and absorb liquids efficiently. Always use clean cloths to avoid reintroducing contaminants to the board surface.
Compressed Air
A can of compressed air is crucial for removing loose debris, dust, and drying small areas of the board. It’s particularly useful for clearing corrosion particles after mechanical cleaning and for ensuring no moisture remains in crevices or under components. When using compressed air, always hold the can upright and use short bursts to avoid the release of propellant liquid onto the board. Maintain a safe distance from the board surface to prevent damage from the high-pressure air stream.
Additional Equipment
To ensure safe and effective cleaning, several additional pieces of equipment are necessary:
- Protective Gloves: Nitrile or latex gloves protect your skin from cleaning chemicals and prevent oils and salts from your skin from contaminating the PCB surface. Choose powder-free gloves to avoid leaving residues on the board.
- Safety Glasses: Eye protection is crucial when working with cleaning solutions and when using compressed air, which can cause particles to become airborne.
- Well-ventilated Workspace: Proper ventilation is essential when working with volatile cleaning agents like isopropyl alcohol. A well-ventilated area helps dissipate fumes and reduces the risk of inhaling harmful vapors.
- ESD-safe Work Surface: An anti-static mat or work surface helps prevent electrostatic discharge, which can damage sensitive electronic components during the cleaning process.
- Magnifying Glass or Microscope: For inspecting small areas of the board and ensuring thorough cleaning, a magnifying glass or digital microscope can be invaluable.
- pH Testing Strips: When using acidic or alkaline cleaning solutions, pH testing strips can help ensure the board is properly neutralized after cleaning.
Methods for Cleaning Corrosion
Cleaning corrosion from printed circuit boards requires a methodical approach and careful execution. The choice of cleaning method depends on the severity and type of corrosion, as well as the specific components and materials present on the board. In this section, we’ll explore various techniques for cleaning PCB corrosion, from gentle methods suitable for light contamination to more aggressive approaches for severe corrosion.
Preparation
Before beginning any cleaning process, proper preparation is crucial to ensure safety and effectiveness. Start by powering down and disconnecting the device, ensuring it is completely powered off and unplugged from any power source. Remove batteries and disconnect any other power supplies to prevent short circuits during the cleaning process. Carefully disassemble the device to access the affected PCB, taking note of the assembly process for correct reassembly later. Conduct a thorough visual inspection of the board under good lighting, possibly using a magnifying glass or microscope, to identify areas of corrosion. Document the board’s condition before cleaning for comparison after cleaning and future reference. Finally, set up your cleaning area in a well-ventilated space, laying out all necessary tools and materials, and ensuring proper lighting and magnification are available.
Compressed Air Cleaning
For light dust and minor contamination, compressed air can be an effective first step. Hold the can of compressed air upright to prevent liquid propellant from escaping, and use short, controlled bursts of air, keeping the nozzle several inches away from the board surface. Pay special attention to areas between components and in crevices where dust might accumulate, working methodically across the board to ensure all areas are addressed. This method is particularly useful for removing loose debris and can help reveal areas that may require more intensive cleaning. However, it’s important to note that compressed air alone is usually not sufficient for removing actual corrosion products.
Isopropyl Alcohol Cleaning
For light to moderate corrosion and general cleaning, isopropyl alcohol is effective. Pour a small amount of high-purity (90% or higher) isopropyl alcohol into a clean container. Dip a soft-bristled brush or cotton swab into the alcohol, ensuring it’s damp but not dripping, and gently scrub the corroded areas in small circular motions. Be careful not to apply excessive pressure, which could damage components or lift traces. For stubborn areas, allow the alcohol to sit on the corrosion for a few moments before scrubbing. Use fresh cotton swabs or brush areas as needed to avoid redepositing contaminants. After cleaning, use compressed air to remove any loose particles and aid in drying, and allow the board to air dry completely in a clean, dust-free environment. This typically takes 15-30 minutes, depending on ambient conditions. Isopropyl alcohol is effective for removing many types of contaminants and light corrosion, but for more severe corrosion, additional methods may be necessary.
Baking Soda Paste Cleaning
For moderate to heavy corrosion, particularly when dealing with acidic corrosion products, baking soda paste is useful. In a small, clean container, mix baking soda with small amounts of distilled water to form a thick paste, similar in consistency to toothpaste. Apply the paste to corroded areas using a cotton swab or soft brush, ensuring the paste covers the corrosion completely. Allow the paste to sit on the affected areas for 15-20 minutes to neutralize acidic corrosion products. Using a soft-bristled brush, gently work the paste into the corroded areas using small, circular motions, being patient and thorough but avoiding excessive pressure. Rinse the area thoroughly with distilled water, using a clean cotton swab or soft brush to help remove all the paste. Use compressed air to blow away excess water, paying particular attention to areas under and between components, and allow the board to dry completely. You may use a lint-free cloth to pat dry accessible areas, followed by air drying for at least an hour. This method is particularly effective for neutralizing and removing acidic corrosion products, with the mild abrasive nature of the baking soda helping in mechanical removal of corrosion without damaging the underlying metal.
Vinegar Cleaning (Use with Caution)
For specific types of corrosion, particularly those involving alkaline compounds, vinegar can be effective. Mix equal parts white vinegar and distilled water in a clean container. Using a cotton swab, apply the diluted vinegar solution directly to the corroded areas, being very precise in your application to avoid spreading the acidic solution to unaffected areas. Allow the solution to sit for no more than 1-2 minutes, as vinegar’s acidity can potentially cause further corrosion if left too long. Gently scrub the area with a soft brush or cotton swab, and immediately rinse the area thoroughly with distilled water to remove all traces of vinegar. Follow up with a baking soda paste application (as described in the previous method) to neutralize any remaining acidity, rinse again with distilled water, and dry thoroughly. This method should only be used under expert guidance and for specific types of corrosion, as the acidity of vinegar, while effective for certain corrosion products, can potentially cause damage if not used correctly.
Post-Cleaning Steps
After completing the cleaning process, conduct a thorough inspection using magnification to carefully inspect the cleaned areas for any remaining signs of corrosion or residue from the cleaning process. Use compressed air to remove any loose debris that may have been dislodged during cleaning, and allow the board to dry completely in a clean, dust-free environment. For critical applications, consider using a low-temperature oven (around 50°C/122°F) to ensure all moisture is removed, but never exceed the maximum temperature rating of the board’s components. Consider applying a conformal coating or other protective measures to prevent future corrosion, especially if the board will be exposed to harsh environments. Finally, carefully reassemble the device and conduct thorough functional testing to ensure all systems are operating correctly.
Safety Precautions and Warnings
Throughout the cleaning process, keep these safety considerations in mind: always work in a well-ventilated area, especially when using volatile cleaning agents; wear appropriate personal protective equipment, including gloves and eye protection; be cautious of static electricity, using an ESD-safe work surface and grounding strap when handling sensitive components; never mix cleaning chemicals unless specifically instructed to do so by an expert; and if you’re unsure about a particular cleaning method or encounter severe corrosion, consult with a professional electronics repair service.
Preventing PCB Corrosion
Preventing corrosion on printed circuit boards is a multifaceted approach that begins at the design stage and continues through manufacturing, storage, and the operational life of the device:
Design and Manufacturing Considerations
The foundation of corrosion resistance is laid during the design and manufacturing phases of PCB production. Material selection is crucial; choose corrosion-resistant materials whenever possible. For example, while copper is the standard for PCB traces, consider using gold plating for critical connections or in harsh environments. The choice of solder mask and other protective coatings also plays a crucial role in corrosion prevention. Applying conformal coatings is one of the most effective ways to protect PCBs from environmental factors that lead to corrosion. These thin, protective layers can be made from various materials such as acrylics, silicones, or urethanes, each offering different levels of protection and flexibility. When selecting a conformal coating, consider factors such as the operating environment, temperature range, and any potential need for rework. Implement design features that minimize moisture accumulation, such as avoiding sharp corners in trace design, using tear-drop pads to improve adhesion, and ensuring adequate spacing between traces to prevent electrolytic corrosion. Implement strict quality control measures during PCB manufacturing, including thorough cleaning to remove flux residues and other contaminants, proper curing of solder mask and conformal coatings, and inspection for defects that could lead to corrosion vulnerability.
Environmental Control
Controlling the environment in which PCBs operate is crucial for preventing corrosion. Maintain relative humidity levels below 60% in areas where PCBs are stored or operated, and consider using desiccants or dehumidifiers in high-humidity environments. Minimize temperature fluctuations, which can lead to condensation, and ensure proper sealing and moisture protection measures are in place if temperature cycling is unavoidable. Implement air filtration systems in industrial or polluted environments to reduce exposure to corrosive gases and particulates. Use sealed or vented enclosures appropriate for the operating environment, and consider IP-rated enclosures for outdoor or harsh environments to provide protection against dust and moisture ingress.
Proper Handling and Storage
Correct handling and storage practices are essential for maintaining the integrity of PCBs. Use anti-static bags or containers for storing and transporting PCBs to protect against electrostatic discharge, which can damage protective coatings and make boards more susceptible to corrosion. Always handle PCBs by their edges to avoid transferring oils and contaminants from skin onto the board surface, and use gloves when necessary, especially in clean room environments. Store PCBs in cool, dry environments with stable temperatures, using moisture-barrier bags with desiccants for long-term storage, particularly for boards with moisture-sensitive components. Implement a first-in, first-out (FIFO) inventory system to ensure older boards are used before newer ones, reducing the risk of corrosion during extended storage periods.
Regular Maintenance
Proactive maintenance can catch corrosion issues early and prevent their progression. Implement a regular schedule for visual inspection of PCBs, especially in critical applications or harsh environments, looking for signs of discoloration, white or green deposits, or any changes in the appearance of metal surfaces. Regularly remove dust and debris using compressed air or gentle brushing, with more frequent cleaning in dusty environments. Conduct periodic functional tests to detect any degradation in performance that might indicate corrosion issues. Maintain detailed records of inspections, cleaning, and any observed changes in board condition to help identify patterns or recurring issues.
Moisture Protection
Protecting PCBs from moisture is critical in preventing corrosion. Use waterproof or water-resistant enclosures in environments where liquid exposure is possible, ensuring that any openings for cables or ventilation are properly sealed. Apply silicone sealants or potting compounds to vulnerable areas such as cable entry points or around sensitive components. Implement proper ventilation to prevent condensation, possibly using Gore-Tex vents that allow air exchange while preventing liquid ingress. In critical applications, consider incorporating moisture sensors that can alert operators to potentially dangerous humidity levels or water ingress. Apply hydrophobic coatings to PCBs and components to repel water and prevent moisture accumulation, particularly in environments where occasional water exposure is unavoidable.
Component Selection
The choice of components can significantly impact a PCB’s resistance to corrosion. Use high-quality, corrosion-resistant components from reputable manufacturers, as this may increase initial costs but can significantly reduce the risk of corrosion-related failures over the life of the device. Choose batteries with robust casings and leak-resistant designs, and consider using lithium batteries for devices that may be stored for long periods, as they are less prone to leakage than alkaline batteries. Use sealed or encapsulated components where possible, particularly for critical or sensitive parts of the circuit, to provide an additional layer of protection against environmental factors. Choose connectors with gold-plated contacts for critical connections, especially in applications where frequent mating and unmating occur, as gold’s resistance to corrosion helps maintain reliable electrical contact over time. Select components with appropriate thermal characteristics and implement effective heat dissipation strategies, as excessive heat can accelerate corrosion processes and degrade protective coatings.
Addressing Root Causes
To effectively prevent PCB corrosion, it’s crucial to address the root causes rather than just treating symptoms. Conduct a thorough analysis of the operating environment for your PCBs to identify potential sources of corrosive agents, humidity, or temperature fluctuations. When corrosion does occur, perform detailed failure analysis to understand the underlying causes and use this information to improve future designs and prevention strategies. Ensure all materials used in the PCB assembly are compatible with each other and with the expected operating environment, as incompatible materials can lead to unexpected chemical reactions and accelerated corrosion. Continuously refine manufacturing and assembly processes to minimize the introduction of contaminants or defects that could lead to corrosion.
Early Detection Strategies
Identifying corrosion in its early stages can prevent minor issues from becoming major failures. Incorporate visual indicators on PCBs that change color when exposed to moisture or corrosive agents, providing an early warning of potential corrosion issues. Implement circuits that can detect changes in resistance or conductivity that might indicate the onset of corrosion, particularly useful in remote or inaccessible installations. Develop and implement a regimen of regular electrical and functional testing to detect subtle changes in performance that might indicate corrosion-related degradation.
Long-term Benefits of Prevention
Investing in comprehensive corrosion prevention strategies offers significant long-term benefits. By preventing corrosion, electronic devices can operate reliably for much longer periods, reducing replacement costs and electronic waste. Corrosion-free PCBs maintain their designed electrical characteristics, ensuring consistent performance over time. Effective prevention reduces the need for costly repairs and replacements, lowering the total cost of ownership for electronic devices. In critical applications, such as medical devices or aerospace systems, corrosion prevention is essential for maintaining the highest levels of reliability and safety. For manufacturers, producing corrosion-resistant products can significantly enhance brand reputation and customer satisfaction.